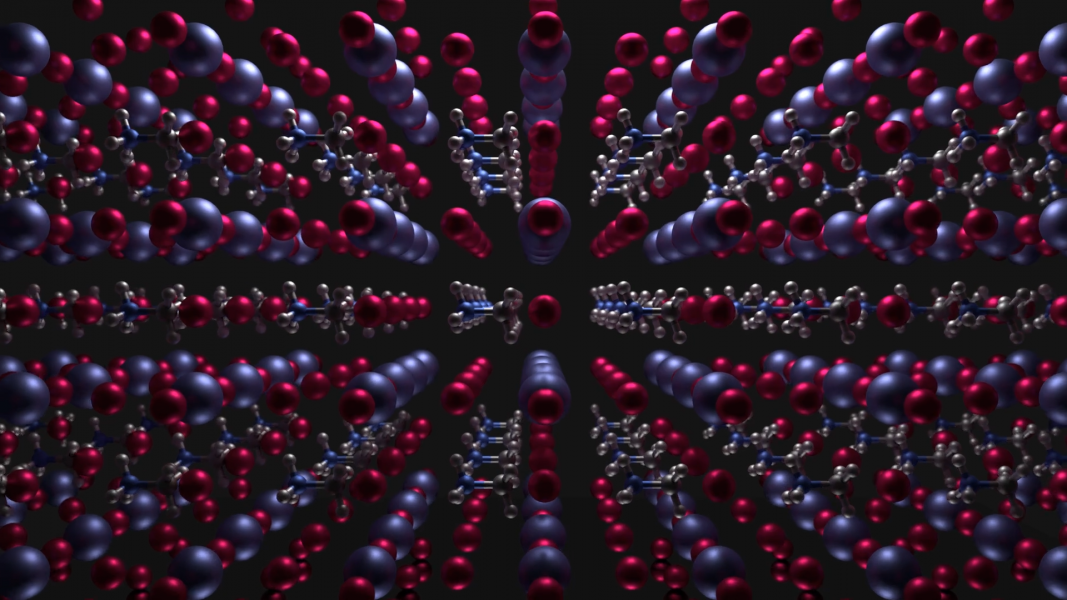
Photovoltaic technology has come a long way, reaching higher power conversion efficiencies than ever before. Recently, organometal halide perovskite has shown remarkable results in solar cell application; however, little is known about the microstructural properties that give rise to their remarkable performance.
In a communication in Advanced Materials, University of Tokyo researchers Tae Woong Kim, Satoshi Uchida, and Hiroshi Segawa, and their co-workers, investigate the structure of organometal halide perovskites, a ubiquitous class of solar cells.
Dr. Tae Woong Kim: “Until now, it has been believed each crystal phase of the perovskite solely exists at its given temperature range. However, in this research, we revealed their crystal phases coexist at room temperature and the coexistence induces self-organized superlattice structure. These evidences overturn the conventional theory. Especially, the existence of the spontaneous superlattice will maximize their potential for wide and diverse applications.”
The authors used transmission electron microscopy (TEM) to elucidate the structure of the perovskite layer in a methylammonium lead iodide solar cell. Unexpectedly, the selected-area electron diffraction pattern does not correspond with a perfect tetragonal crystal structure.
High-resolution TEM confirmed the existence of the non-uniform lattice in the perovskite layer, revealing two distinct diffraction patterns belonging to tetragonal and cubic structures, and providing further evidence that these two phases coexist at room temperature.
An apparent distortion of the cubic lattice by the tetragonal structure causes the iodine atoms to be shifted relative to their simulated positions.
A triple-layer superlattice was also identified, arising from the combination of tetragonal and cubic planes. The diffraction pattern and corresponding line profile clearly show that the superlattice is composed of a tetragonal/cubic/tetragonal stacking sequence.
Prof. Satoshi Uchida: “This new finding was exciting and we are confident for our results because spot patterns of electron diffraction were really beautiful—like a snow crystal or a flower—that indicate clear evidence of the superlattice. I hope this new discovery will lead to the further improvement of the cell performance by controlling the phase boundary of the perovskite.”
To find out more about this crystallographic study of halide metal perovskites, please visit the Advanced Materials homepage.