 Lanthanide ion (Ln3+)-doped upconversion nanoparticles (UCNPs) are able to absorb near-infrared (NIR) photons and convert such low energy excitations into shorter wavelength emissions. Due to this unique property, UCNPs have emerged as a new class of materials in a wide range of applications, such as biosensing, chemical sensing, in vivo imaging, drug delivery, photodynamic therapy and photoactivation.
Lanthanide ion (Ln3+)-doped upconversion nanoparticles (UCNPs) are able to absorb near-infrared (NIR) photons and convert such low energy excitations into shorter wavelength emissions. Due to this unique property, UCNPs have emerged as a new class of materials in a wide range of applications, such as biosensing, chemical sensing, in vivo imaging, drug delivery, photodynamic therapy and photoactivation.
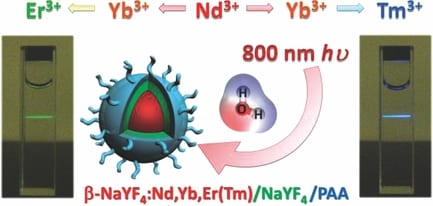
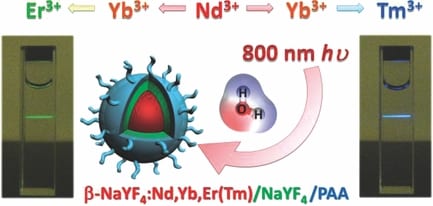
However, one existing limitation of the most commonly used Yb3+-sensitized UCNPs is their physically unalterable excitation band centered at 980 nm, overlapping at the maximum absorption peak of water molecules. Because cells and tissues withhold 980 nm radiation and concomitantly induce heat damages, this becomes problematic for the application of UCNPs in H2O-rich live systems. In particular, such a heat effect is likely to be more severe in certain experiments that require higher power density and longer–term excitation, such as single-nanoparticle imaging or longitudinal deep-tissue imaging. In contrast, in the tissue optical window, the local minima of water absorption is at around 800 nm, which is considered to be the ideal excitation wavelength with the least impact on biological tissues.
To overcome this problem, a team led by Dr Gang Han at University of Massachusetts-Medical School, have developed cascade-sensitized 800 nm excited tri-doped UCNPs. This novel class of UCNPs employ Nd3+ as an 800 nm photon sensitizer and Yb3+ as a bridging ion, and show strong upconversion emission without photobleaching. Such new materials have been demonstrated to outperform classical 980 nm excited UCNPs in regard to significantly decreased NIR photon absorption in water and decreased laser-induced water heating effects.
This new concept is of considerable practical importance and will pave the way for the development of new generations of UCNPs with better biocompatibility and exciting penetrability.