Mesenchymal Stromal Cells (MSC) are self-renewing multipotent cells that can differentiate into a variety of cell types including bone, muscle and fat. Because of their potential for giving rise to cells of diverse lineages, MSCs have been used in medical treatments and hold great promise for use in regenerative medicine and immunomodulation. However, clinical use of MSCs is currently limited due to insufficient yield, invasive collection methods and poor standardization of isolation and characterization methods.
Since MSCs obtained from young donors proliferate better, umbilical cord (UC) is a preferred source of MSCs (UC-MSCs) especially due to the safety, ease and non-invasive nature of the method of obtaining these tissue from young donors. While several methods to isolate and characterize UC-MSCs have been published, there is a lack of a standard within the field causing a general lack of uniformity and reproducibility with respect to UC-MSC isolation and QA methods across labs. To address this issue, Mark Weiss’s group at Kansas State University has detailed a comprehensive series of protocols for efficient isolation, expansion and characterization of UC-MSCs in the latest issue of Current Protocols in Stem Cell Biology.
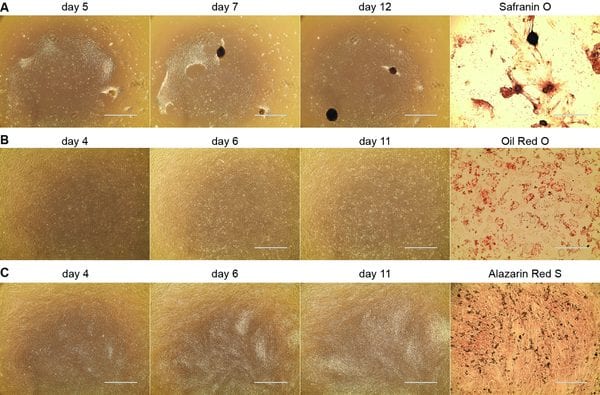
MSC growth during differentiation.
In CPSC Unit 1F.18, Weiss et al. address issues such as use of xenogeneic agents in culture media and contamination risk introduced by manual mincing of the cord, which limited the clinical value of the resultant UC-MSCs and reduced yield. The new protocols describe a culture medium that eliminates use of animal products and outline a method to characterize the isolated UC-MSCs per the International Society of Cell Therapy (ISCT) minimum definition. The authors include several helpful, individual protocols to aid quality control and ensure that the UC-MSCs are characterized thoroughly while improving yield. A primary advantage of the isolation protocol is that it is scalable. The Anticipated Results section even provides a fairly good idea of what yield users should expect when using this method and images of the characteristics of the UC-MSCs.
This is unit is an important step to further the conversation regarding the uniformity and reproducibility of UC-MSC isolation methods leading to rapid translation into clinical manufacturing and testing. This protocol also provides a reference guideline for labs that need a GMP method to isolate and characterize UC-MSCs that fit ISCT minimum definition.