Computational chemistry has been traditionally relegated to a small box within a paper to help explain a mechanism or provide some other insights about a reaction that had been performed in the lab. But computational chemistry is way more than just this — there is more to offer.
With increasing computational capacity and new techniques, over the last two decades computational methods have provided a more economic approach to exploring different chemical systems. However, even in light of this benefit, experimental screening is still very much king in most chemists’ minds — experiments are run first and then computational investigations are performed afterward to complement experimental findings.
But computational design can not only save time and money, it can be used in designing new catalysts and substrates based on theoretically proposed models.
One area that is of particular interest, especially in the present climate, is the application of computational modelling in uncovering catalysts to enable efficient, sustainable processes, such as carbon dioxide recycling, making biodegradable plastics, fertilizers, disinfectants more sustainably, and obtaining a wide range of pharmaceutical compounds.
Another lies in creating catalysts for the pharmaceutical industry. In nature, some molecules exist as mirror-images of each other — they are non-superimposable, like our left and the right hands. These are called chiral molecules, and each form is called an enantiomer. The intriguing thing is that while one enantiomer of a chiral drug may be a helpful medicine, the other enantiomer of the same molecule may be not only inactive but can also be toxic.
Ibuprofen, for one, has two enantiomeric forms where one is pharmacologically active and the other has no anti-inflammatory effect. While this example might be benign, the differences between chiral forms can be very dramatic, such as in the case of Thalidomide, a sedative withdrawn from the market in the 1960s. One of its enantiomers has beneficial, sedative effects, whereas the other causes birth defects.
The importance of producing one enantiomer over the other could not be more important. This is an area of catalysis known as asymmetric organocatalysis and its relevance is difficult to overstate since the majority of the new drugs approved by the FDA are chiral molecules, the properties which — including inactive enantiomers — must be known before the drug can be approved.
Computational modelling has the capability to reduce the amount of time and resources that go into developing a new catalyst for these applications, but its “skills” are vastly underutilized. Can catalysts be designed in silico without experimentation?
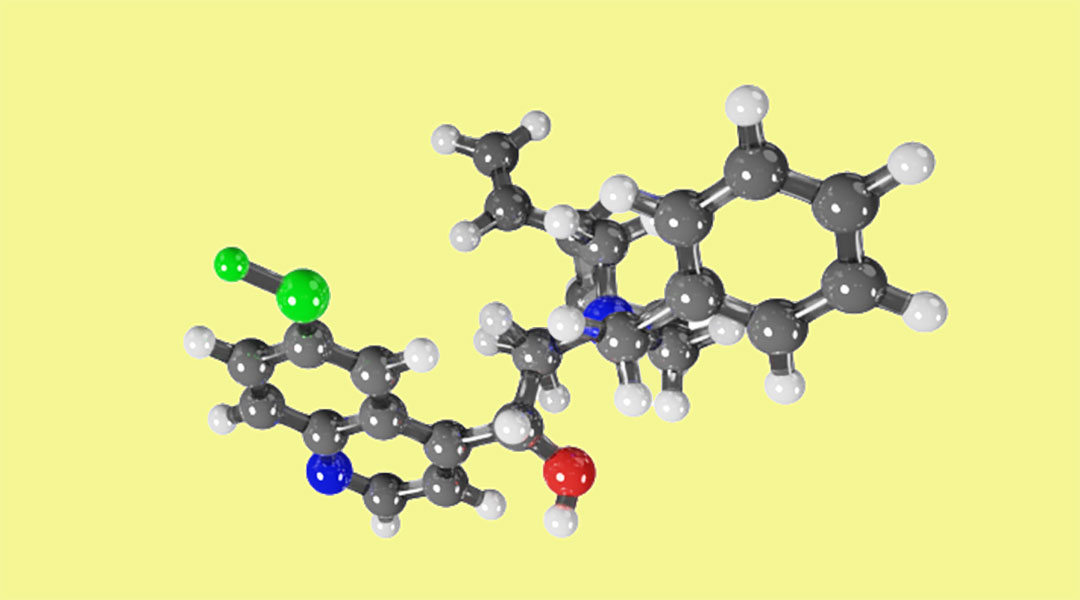
Examples from our own work point to this being the case. In one recent study, we applied computational methods to study the interactions of one particular bifunctional phase transfer catalyst widely applied in organic reactions. Phase-transfer catalyst systems work between two liquid immiscible phases where the catalyst transfers between the phases, driving product formation in one phase and then becomes regenerated in the other phase. We analyzed the interaction between the bifunctional phase transfer catalyst and four different anions of interest in organocatalysis and provided a definitive binding mode.
It was discovered that the binding mode between the catalyst and the reactants was not the one described in the literature, as determined by experimentation. The reasoning behind why the catalyst provided one enantiomer had, until that point, been unjustifiably described which led to a poor catalyst design, since there are no well-defined attractive interactions between the catalyst and the reactants. Understanding this new binding mode and analyzing different interactions upon complexation by means of computational chemistry led to small change in one group within the catalyst, and the theoretical selectivity was increased to 90%.
This is just one simple example but clearly outlines how computation can be used to help in making chemistry more efficient, allowing us an alternative perspective and to re-evaluate what we truly know about the reactions we are running.
I think is fair to say that the era of computational catalyst design has arrived, and it is here to stay.
Reference: Iñigo Iribarren, Marianne Rica Garcia and Cristina Trujillo, Catalyst design within asymmetric organocatalysis, WIREs Computational Molecular Science (2022). DOI: 10.1002/wcms.1616