The diagnosis of diseases is an ongoing healthcare challenge. Unravelling the dynamic protein secretion process of single cells would provide valuable insight into human diseases, which could lead to improved therapies.
In their article in Small, Professor Hatice Altug and colleagues from École Polytechnique Fédérale de Lausanne (EPFL), and their co-workers from RMIT University and University of Lausanne demonstrate a label-free optofluidic nanobiosensor that enables real-time analysis of single-cell cytokine secretion.
A plasmonic nanohole array biosensor was constructed by coating a quartz wafer with a gold film containing billions of nanoholes. The sensitivity of the array outperforms state-of-the-art nanoplasmonic biosensors.
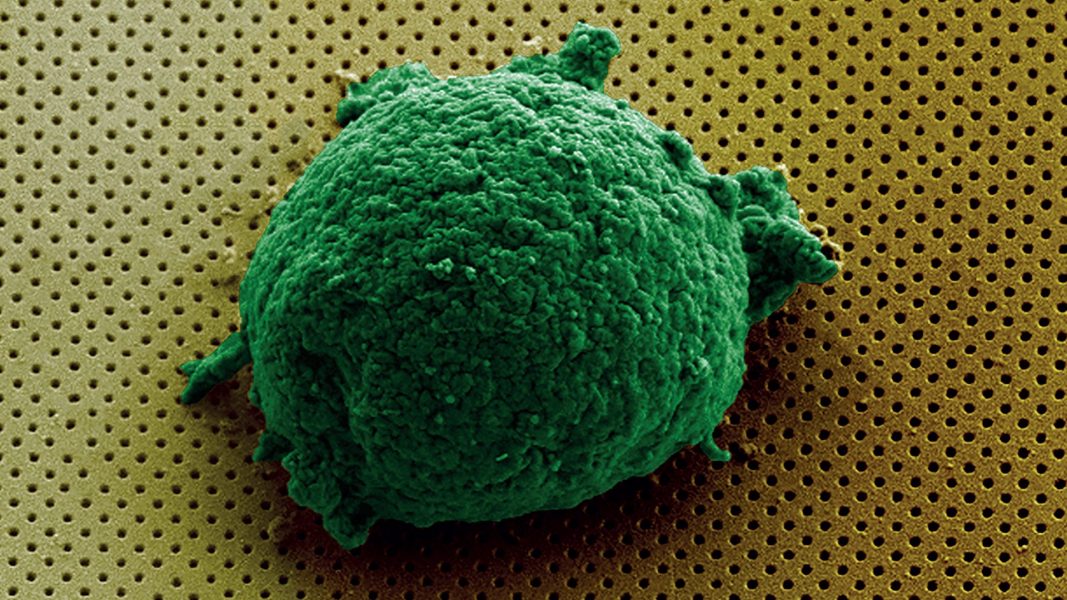
The surface of the biosensor was functionalized to promote cell attachment and cytokine detection. Cell immobilization on the surface was confirmed by scanning electron microscopy.
A microfluidic device was fabricated, which integrated the biosensor and provided a small incubation chamber controlled by microvalves. This unique lab-on-a-chip system enabled protein secretion from a single cell to be detected in a precise and accurate manner.
To find out more about this innovative optofluidic nanobiosensor, please visit the Small homepage.