 A scientific team in Hong Kong has developed a new method for detecting the lethal bird flu virus quickly, using a new class of materials called upconversion nanoparticles. These nanoparticles, a billionth of a meter in size, are relatively low-cost, extremely sensitive, and fast acting. These attributes will enable health officials to curb poultry outbreaks and treat affected human beings much more rapidly, thus preventing both animal and human pandemics and saving lives.
A scientific team in Hong Kong has developed a new method for detecting the lethal bird flu virus quickly, using a new class of materials called upconversion nanoparticles. These nanoparticles, a billionth of a meter in size, are relatively low-cost, extremely sensitive, and fast acting. These attributes will enable health officials to curb poultry outbreaks and treat affected human beings much more rapidly, thus preventing both animal and human pandemics and saving lives.
Avian flu outbreaks in developing countries in Asia have sickened and killed many people and caused millions of dollars in economic damage in the last decade. New pandemics continue to be a threat, as the virus mutates into more lethal subtypes that spread rapidly among poultry and can be transmitted to humans.
So far, the early, rapid detection and accurate diagnosis of the avian flu virus has remained a challenge. Despite some progress, conventional detection methods require developed infrastructure, and are expensive, highly chemical specific, and labor intensive, with time-consuming virus isolation. The enzyme-linked immunosorbent assay method, known as ELISA, is rapid, but its sensitivities are relatively low. The target amplification methods, such as reverse transcription- polymerase chain reaction (or PCR) for avian detection require expensive equipment and highly trained personnel, and are susceptible to contamination during the amplification process.
The use of upconversion nanoparticles as biosensors solves these problems.
How Upconversion Nanoparticles Work
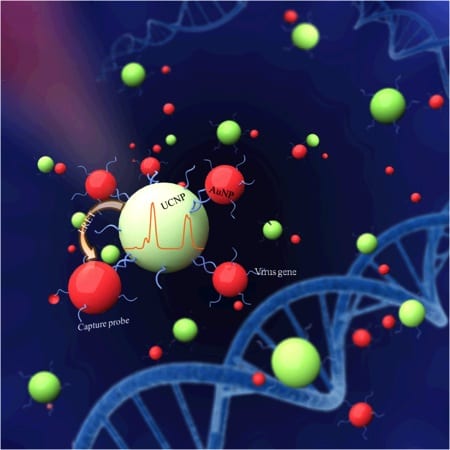
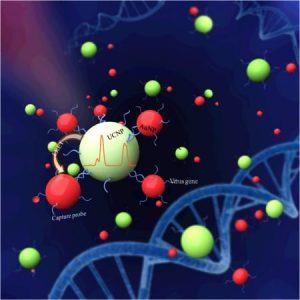
Upconversion nanoparticles are a new class of materials that can convert near-infrared light into visible ultraviolet emission, by sequentially absorbing multiple photons. The nanoparticles used in the new method are lanthanide based. The photoluminescence that accompanies the upshift can be measured, and if the virus is present in the sample, it will reduce the measured photoluminescence.
The design of the Hong Kong experiment coupled lanthanide-based upconversion nanoparticles as a donor with gold nanoparticles as an acceptor. The gold particles were mixed with avian flu virus material (a hemagglutinin gene segment). When near-infrared laser light at 980 nanometers was used to excite the upconversion nanoparticles, the gold nanoparticles absorbed the higher energy ultraviolet light emission from the upconversion nanoparticles, and the change in total photoluminescence intensity in the solution could be measured. The upconversion process, which transforms low-energy near-infrared light into visible light, does not exist naturally in live animals, ruling out contamination in the experiment. The upconversion process demonstrated a superior capability for detecting the virus with greater sensitivity than other methods.
Luminescence Resonance Energy Transfer
This new luminescence resonance energy transfer (or LRET) biosensor was demonstrated with the H7 subtype of avian flu A. The researchers designed a method of conjugating the H7 hemagglutinin gene oligonucleotides (single-stranded DNA molecules) onto the gold nanoparticles. The luminescence resonance energy transfer process occurs when laser excitation interacts with complementary oligonucleotides. The gold nanoparticles conjugated with avian flu resonate at a frequency very close to that of the ultraviolet output of the upconversion nanoparticle, thus quenching the total bioluminescence of the solution. The H7 virus target can be quantified down to a lower limit of 7 picometers.
Because of its simplicity, fast action, and high sensitivity and selectivity, this new upconversion nanoparticle biosensor is a valuable front-line diagnostic tool that can be made available for cost-effective use in developing countries.