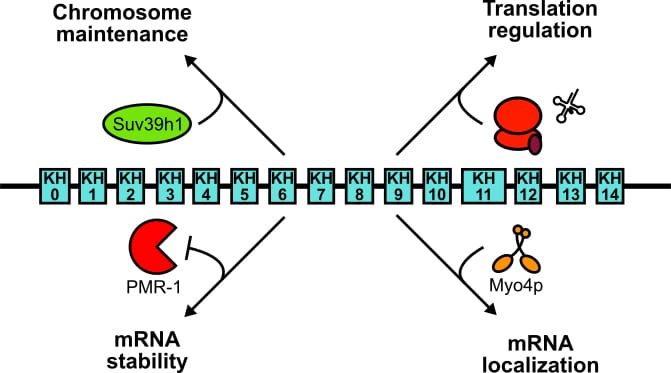
Many cellular processes are coordinated by the action of RNA-binding proteins (RBPs), which bind mRNAs at specific recognition elements to control their fate. The vigilin protein family is conserved from yeasts to humans and are characterized by their unusual architecture. Comprising 14-15 nucleic acid-binding domains (so called “KH” domains), vigilins belong to the largest RBPs known to date. Although they are recognized as RNA binders today, human vigilin was first described 30 years ago as a high-density lipoprotein-binding protein. Studies that followed have shown vigilin to be involved in many aspects of RNA biology as well as chromosome biology, which is supported by its presence both in the nucleus and the cytoplasm. Despite an abundance of research, our understanding of the vigilins’ molecular workings remain obscured in part by the multifaceted nature of their functions and a lack of parallel studies across organisms.
To mark 30 years of vigilin research, Matthew HK Cheng and Ralf-Peter Jansen summarize and discuss the major findings from research on the vigilin protein family in an advanced review published in WIREs RNA. They provide a brief historical outline and context to vigilin’s many names as well as a consolidated overview of vigilins’ molecular functions in the nucleus and in the cytoplasm. These functions are presented in relation to vigilins’ protein structure and the various interacting factors, highlighting the parallels and differences among simple unicellular and complex metazoan organisms. Finally, they outline the most pressing concerns and outstanding questions that lie ahead for the field.
Kindly Contributed by the Authors