Harvard scientists may have discovered that xenon gas has neuroprotective properties, potentially offering a new avenue for combating Alzheimer’s disease, based on promising results from preclinical trials.
“Xenon, a seemingly humble noble gas, can ‘coach’ microglia—our brain’s immune cells—into stepping up their game,” said Oleg Butovsky, associate professor at Harvard Medical School and contributing scientist to the study, in an email. “It nudges [microglia] toward a state where they’re effective at cleaning up toxic protein buildup without causing collateral damage, making it a powerful ally in combating Alzheimer’s disease.
Alzheimer’s disease is one of the most common neurodegenerative diseases globally and is characterized by abnormal buildup of amyloid and tau proteins Under normal conditions, the precursor to amyloid called APP plays a role in cell development and function, while tau helps stabilize the microtubules that support neurons.
In patients with Alzheimer’s, abnormal amyloid and tau proteins accumulate over time due to improper folding or aggregation. This buildup kills neurons and their connections, reducing signalling and brain function. Over time, areas of the brain shrink, and clinical symptoms of memory loss, poor decision-making, and mood changes manifest.
Despite an increase in global prevalence, there is a need for additional and more effective treatments for Alzheimer’s as scientists have struggled to understand the complex mechanisms behind the disease.
Thinking outside the box when treating Alzheimer’s disease
Current therapeutic strategies focus on breaking down this protein buildup to slow disease progression, but these are not fully effective and there is much debate about why.
One important concern is that treatments that target amyloid or tau are being started too late — since the changes in the brain begin years before clinical symptoms arise, the damage may be too severe for treatments to work effectively.
Given the lack of successful strategies targeting amyloid and tau residues, Butovsky and his team shifted focus to a new target: brain cells called microglia. Butovsky described microglia as the “brain’s cleanup crew,” whose job is to remove dead cells, microbes, and protein buildup. However, when activated by the buildup of amyloid and tau proteins, they can become dysregulated because these abnormal protein deposits are seen as damaged, which prompts the microglia to act. Typically, microglia would respond by clearing these protein buildups and repairing any damage.
In Alzheimer’s disease, this process goes awry. “In Alzheimer’s, they can become either too lazy or overly aggressive, both of which spell trouble,” Butovsky explained.
Microglia also play an essential role in mediating inflammation and maintaining neuron networks, which means any changes to how they function can cause additional negative brain alterations and neurodegeneration.
Enter xenon
Intrigued by xenon’s rare ability to cross the blood-brain barrier and its neuroprotective effects on microglia in brain injury models, Butovsky and his team wondered if it could do the same for Alzheimer’s disease. In preclinical brain injury studies, xenon was shown to regulate inflammation and reduce damage to neurons — raising the possibility that it could help protect the brain from Alzheimer’s-related decline.
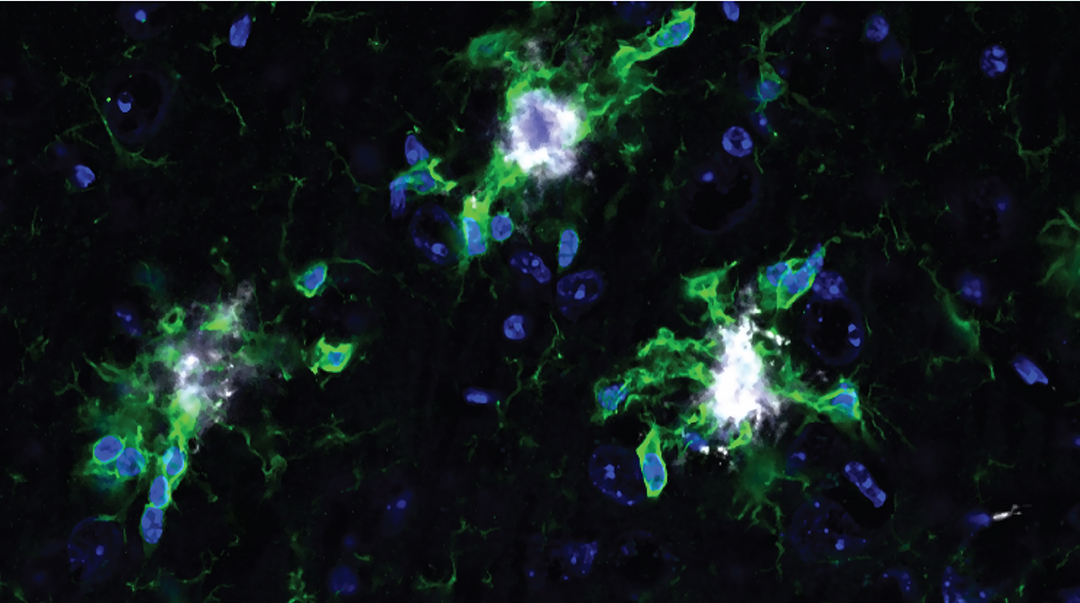
Testing their hypothesis in mouse models of Alzheimer’s, the team found that inhaled xenon helped restore microglia to a more balanced state, allowing them to return to cleaning up dead cells and proteins. The team thinks xenon helps restore balance by influencing immune signals in the brain, particularly a molecule called IFN-γ, which plays a key role in inflammation. However, they say its effects are likely more complex, and further research is needed to fully understand how it works.
Xenon also reduced the amount of abnormally swollen neurons and lowered inflammation levels in the brain. Clinically, this translated into a return to more normal behaviors in mice who had received Xenon treatment compared to those who had not.
“Xenon acts like a skilled manager, guiding these cells into a balanced ‘just right’ state,” said Butovsky. “It helps them clear out harmful amyloid plaques while reducing inflammation, which can otherwise damage healthy neurons. This dual action addresses two major culprits of Alzheimer’s disease.”
A game changer for neurodegenerative diseases?
As David M. Holtzman, professor of neurology at Washington University School of Medicine and co-lead of the study, pointed out, researchers increasingly focus on microglia in Alzheimer’s research. “There are a number of other therapies in development that target microglia in ways different from Xenon. Some examples include both agonist antibodies and small molecule agonists of TREM2,” he explained.
“Compared to other microglia-targeting strategies, such as TREM2 agonists, Xenon provides broader modulation with a well-established safety profile from its clinical use in anesthesia, positioning it as a promising therapeutic candidate,” Butovsky added.
The next step is a Phase I clinical trial set for early 2025, where researchers will assess the safety and optimal dosage of xenon gas in humans. This trial marks the beginning of what the team hopes will be a series of successful studies. However, challenges lie ahead.
“[These] include ensuring consistent effects across diverse populations, scaling the treatment for widespread use, and navigating the regulatory landscape,” said Butovsky. “But with the support of this incredible collaborative team, we are optimistic about overcoming these hurdles.”
Further research will also take time, and the team notes that if trials are successful, patients may begin to see benefits from the treatment in the next 3 to 5 years. “It’s an exciting time for science, and we are eager to see how this innovative approach might bring hope to millions of patients and families worldwide,” remarked Butovsky.
He emphasized that the success of the research has been driven by a mix of junior and senior scientists and physicians working together, with diverse expertise playing a crucial role. “Their diverse expertise and teamwork made it possible to uncover the therapeutic potential of xenon,” he concluded.
Reference: Oleg Butovsky, et al., Inhaled xenon modulates microglia and ameliorates disease in mouse models of amyloidosis and tauopathy, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adk3690
Feature image: Brain cells treated with xenon in mouse models of Alzheimer’s. Microglia in green and amyloid-beta plaques in white. Credit: Oleg Butovsky, et al.