Scientists are developing a solution to help cure chronic wounds that persist due to disrupted healing processes associated with diabetes.
“The diabetic wound healing is challenging due to the sabotaged delicate balance of immune regulation via an undetermined mechanism, so it is crucial to decipher multicellular signatures underlying diabetic wound healing and seek therapeutic strategies,” said Zheng Li at Peking University.
Hydrogels are jelly-like materials that hold large amounts of liquid. As such, they stay moist and flexible, making them excellent for wound care. They can also absorb any fluids exuded and completely fill wounds, keeping harmful bacteria out.
Zwitterionic hydrogels take this a step further — made from materials that have both positive and negative charges, their dual charges let them attract both positively and negatively charged molecules. In past experimental studies, these hydrogels have been shown to be effective in healing diabetic wounds, but how they worked wasn’t clear.
Immune cells and wound healing in diabetes
To try to unravel the mechanism, Li and her colleagues Yongsheng Zhou, also at Peking University, and Xiong Pu at the CAS Center for Excellence in Nanoscience, studied the immune response at the cellular level under their influence.
For wounds to heal well, the immune response must be precisely orchestrated. Various types of immune cells must be called in, do their jobs, then be removed and replaced once their task is complete. For example, in the inflammation phase of healing, immune cells remove dead cells and kill bacteria, but if this phase lasts too long it harms healthy tissue and slows growth of new cells.
With diabetes, the inflammation phase of healing starts too late and lingers too long. Wounds that take too long to heal can become infected and cause other complications; better treatments are needed.
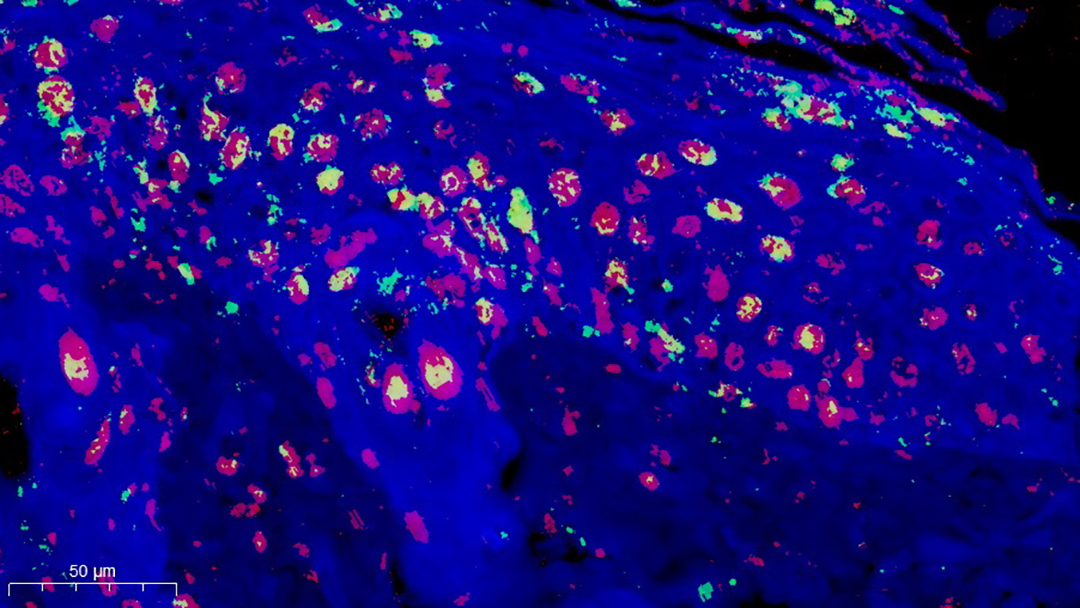
Applying these hydrogels to wounds of diabetic mice, the researchers found that the wounds healed faster and with less inflammation. To figure out why, the team used single-cell RNA sequencing, reading the genetic material in individual cells.
This technique helps the researchers identify individual immune cells and which genes were activated, giving them a clear picture of how the immune system was responding. The group found that the zwitterionic hydrogels boosted certain immune cells called Ccl3+ macrophages and suppressed others — S100a9+ neutrophils.
Neutrophils are some of the first immune cells recruited to a wound. They help remove bacteria, and also generate inflammation. When their work is done, they need to be removed for inflammation to subside.
Macrophages coordinate the skin repair process, keep neutrophils away from healthy tissue, and help remove neutrophils when their work is done. By boosting macrophages and suppressing neutrophils, the hydrogel balanced the immune environment, reduced inflammation, and promoted growth of new tissue.
Suppressing pathogen-catching “NETs”
The hydrogel had another beneficial effect: it stopped excessive production of a wound-healing component called neutrophil extracellular traps (NETs).
NETs are networks of fibers studded with proteins that kill bacteria. In healthy wounds, only a few NETs develop, removing pathogens but not harming healthy tissues. In diabetic wounds, too many NETs are formed, leading to excessive inflammation and damaged tissue.
The researchers used a very high-resolution type of microscopy called scanning electron microscopy to count NETs inside cells. They found that wounds treated with the zwitterionic hydrogel showed substantially fewer NETs, which they think is linked to improved healing. It’s not yet clear why the hydrogel suppressed NET formation, only that it had a beneficial effect.
The group’s findings reveal both a potential pathway for development of new treatments and help illuminate how healing is disrupted in diabetes. Studying how zwitterionic hydrogels can boost the immune response may help in finding ways to improve healing of diabetic wounds.
“We are further studying zwitterionic hydrogels with simpler components and better effects for tissue repair and seeking clinical applications,” said Li.
Reference: Zheng Li, Longwei Li, Muxin Yue, Qingyu Peng, Xiong Pu, Yongsheng Zhou, Tracing Immunological Interaction in Trimethylamine N-Oxide Hydrogel-Derived Zwitterionic Microenvironment During Promoted Diabetic Wound Regeneration, Advanced Materials (2024). DOI: 10.1002/adma.202402738